Expanding a product line globally requires navigating different regulatory requirements in each region. Since approval in one country doesn’t guarantee compliance in another, companies must understand the distinct processes of major regulatory bodies.
Key agencies include the FDA (United States), EMA (European Union), MHRA (United Kingdom), PMDA (Japan), and TGA (Australia).
The United States: FDA (Food and Drug Administration)
The FDA is often viewed as the global gold standard for regulatory oversight. As the agency responsible for protecting public health in the United States, its scope is vast, covering food safety, tobacco products, dietary supplements, prescription and over-the-counter pharmaceutical drugs, vaccines, biopharmaceuticals, and medical devices.
Key Characteristics of FDA Regulation
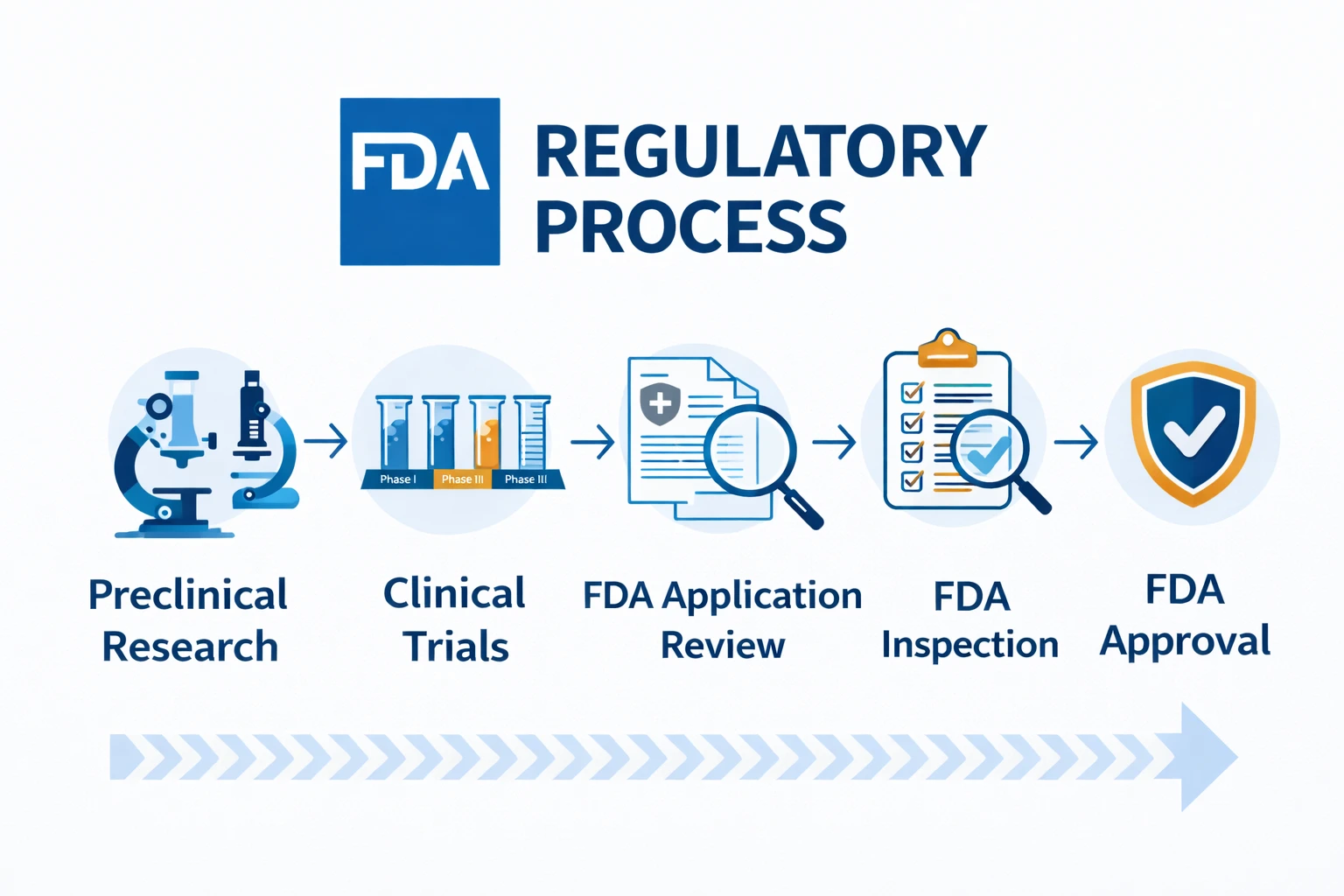
The FDA operates under the Code of Federal Regulations (CFR), specifically Title 21. For medical device manufacturers, the path to market usually involves either a 510(k) premarket notification (demonstrating substantial equivalence to an existing device) or a Premarket Approval (PMA) for high-risk novel devices.
For pharmaceuticals, the New Drug Application (NDA) process is rigorous. The FDA is known for its intense scrutiny of clinical trial data and manufacturing processes (cGMP).
They conduct thorough facility inspections, often unannounced, to ensure ongoing compliance. Understanding the specific classification of your product is step one, as the FDA’s definition of a “drug” versus a “supplement” or “food” can fundamentally alter your compliance roadmap.
The European Union: EMA (European Medicines Agency)
In the European Union, the regulatory landscape is unique because it involves coordinating across 27 member states. The European Medicines Agency (EMA) is the decentralized agency responsible for the scientific evaluation, supervision, and safety monitoring of medicines.
The Centralized Procedure
Unlike the FDA, which is a single federal body, the EMA coordinates closely with national competent authorities in each member state. For many innovative products—such as cancer treatments, diabetes medications, and treatments for rare diseases—companies must use the “centralized procedure.”
Under this route, a company submits a single marketing-authorization application to the EMA. If the agency’s scientific committee (CHMP) provides a positive recommendation, the European Commission grants a centralized marketing authorization valid in all EU member states.
It is important to note that for medical devices, the landscape has shifted dramatically with the introduction of the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR).
These regulations have increased the burden of proof regarding clinical evidence and post-market surveillance, requiring manufacturers to work with “Notified Bodies” rather than the EMA directly for certification.
The United Kingdom: MHRA (Medicines and Healthcare products Regulatory Agency)
Following the UK’s departure from the European Union (Brexit), the role of the Medicines and Healthcare products Regulatory Agency (MHRA) has evolved. No longer under the umbrella of the EMA, the MHRA now acts as a standalone sovereign regulator for the United Kingdom.
Post-Brexit Independence
The MHRA has established new routes to market to ensure the UK remains an attractive destination for life sciences. One significant change is the transition from the CE mark (used in the EU) to the UKCA (UK Conformity Assessed) mark for medical devices, although the timeline for this transition has seen several extensions to allow industry adaptation.
The MHRA has also introduced the Innovative Licensing and Access Pathway (ILAP), designed to accelerate the time to market for medicines. This pathway allows for earlier engagement between the developer and the regulator, fostering a more collaborative approach to data generation and clinical trial design.
Japan: PMDA (Pharmaceuticals and Medical Devices Agency)
Japan represents one of the largest pharmaceutical and medical device markets in the world, but it is also known for having some of the most stringent regulatory requirements.
The Pharmaceuticals and Medical Devices Agency (PMDA) works in conjunction with the Ministry of Health, Labour and Welfare (MHLW) to handle reviews and safety monitoring.
Focus on Local Population Data
A distinct characteristic of the PMDA is its emphasis on data relevant to the Japanese population. Historically, the agency has required “bridging studies” to demonstrate that foreign clinical data is applicable to Japanese patients, due to potential differences in metabolism and genetic factors.
While the PMDA has harmonized some aspects of its review process with global standards, companies should still expect rigorous reviews of quality and manufacturing standards. The “Sakigake” designation system is Japan’s version of a breakthrough therapy designation, offering priority review for innovative products that address severe unmet medical needs.
Australia: TGA (Therapeutic Goods Administration)
In Australia, the Therapeutic Goods Administration (TGA) regulates medicines, medical devices, and biologicals. The TGA employs a risk-based approach, meaning the level of regulatory control increases with the level of risk the product poses to the consumer.
The ARTG Entry
For a product to be lawfully supplied in Australia, it must be included in the Australian Register of Therapeutic Goods (ARTG). The TGA divides medicines into “registered” (higher risk, rigorous evaluation of safety, quality, and efficacy) and “listed” (lower risk, evaluation of quality and safety).
Australia often leverages assessments from comparable overseas regulators (like the FDA or EMA) to streamline its own approval processes. This “comparable overseas regulator” pathway can significantly reduce evaluation times for companies that have already secured approval in other major jurisdictions.
Best Practices for Global Compliance
Successfully launching a product across these diverse regions requires a strategic, proactive approach. Trying to retrofit a regulatory strategy after product development is complete is a recipe for expensive delays.
Early and Frequent Engagement
Don’t wait until your dossier is perfect to talk to regulators. Most agencies, including the FDA and EMA, offer pre-submission meetings. These sessions allow you to present your testing plan and get feedback on whether your proposed clinical trials will satisfy their requirements.
Embrace Harmonization Standards
Wherever possible, utilize global standards. The International Council for Harmonisation (ICH) for pharmaceuticals and the International Medical Device Regulators Forum (IMDRF) for devices provide guidelines that are accepted by multiple regulatory bodies. Adhering to these guidelines helps create a “core dossier” that can be adapted for different regions with minimal rework.
Leverage Local Expertise

Nuance is everything. A regulation might look clear on paper, but the interpretation by local inspectors can differ.
If you are launching a specialized nutrition product, for example, partnering with a food and beverage regulatory consultant who understands the specific labeling and ingredient claims allowed in that region is invaluable. The same applies to local legal representation for medical device registration.
Conclusion
The regulatory environment is not static; it is a living ecosystem that shifts with political changes, scientific advancements, and public health crises. From the FDA’s rigid safety protocols to the MHRA’s agile post-Brexit pathways, each agency presents a unique set of challenges and opportunities.